Myths and Controversies About Cleaning Silver
I am a full-time mom, part-time researcher, and part-time jewelry designer.

Circa 1920's sterling silver Chinese Export pendant set with mother of pearl and marcasites.
Courtesy of Naiad Necklaces
While many might disagree, I prefer to remove tarnish from my silver jewelry or silverware by chemically reversing the actual tarnish process. I use three simple household ingredients: water, aluminum foil, and baking soda. Try searching for these ingredients and you will find remarkably consistent instructions across several websites: Place your jewelry pieces or silver on top of crumpled aluminum foil and sprinkle baking soda over them generously. Pour boiling water until the jewelry is covered. Wait 10 minutes, remove the jewelry, and rinse it under hot water. Pat until thoroughly dry and let sit out overnight. Repeat as necessary.
This is all straightforward enough, but I found myself wondering, “Why 10 minutes? What if something is really tarnished? Why not let it sit longer?” “And why does the water need to be boiling?” Most websites are pretty adamant on both points: no more than 10 minutes, ever, and yes, the water has to be boiling.
Chemically speaking, tarnish is silver sulfide, and it can be reduced back to silver in a classic redox electrochemical reaction. Tarnished silver (the cathode) is placed in direct contact with an anode (the aluminum) in a saltwater bath (the baking soda solution). The saltwater facilitates the movement of electrons between the silver and aluminum. Since aluminum has a much higher affinity for sulfur atoms than silver, the silver ion is reduced back to silver, and sulfide ions are released. These bind to aluminum to form aluminum sulfide. The aluminum corrodes, and the silver turns shiny.
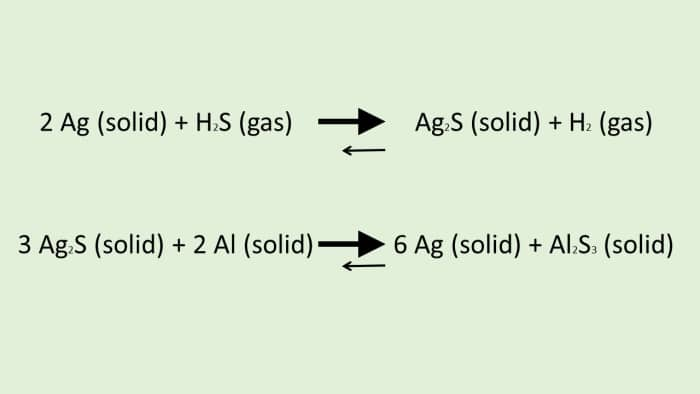
Most of the chemical reaction moves from left to right, as shown by the much larger arrow, but there is still some movement from right to left, as shown by the smaller arrow
Kinetic Reaction Between Silver, Hydrogen Sulfide, and Aluminum
Redox reactions are kinetic. This means that electrons and ions are moving back and forth between the silver and the aluminum. So even though, mostly, the aluminum is being oxidized (corroded) and the silver, reduced, a tiny bit of aluminum is also being reduced and a tiny bit of silver is being oxidized. I could imagine that after 10 minutes, say, maybe the kinetics change so that silver gets corroded more than aluminum. For example, if all the available aluminum gets converted to aluminum sulfide, the kinetics might reverse, and the silver might re-tarnish.
I had a harder time with the boiling water assertion. Sure, boiling water would make the ions move faster, speeding everything up, but surely you could get the same results with more tepid water? This was an important question because sometimes when you rinse delicate jewelry pieces after they have been in boiling water, the sudden temperature change can crack the piece (you should always rinse the jewelry in very hot water to avoid this). Also, if you have stones that have been set with glue, cooler water just might be a little less likely than boiling water to loosen the adhesion. I decided to test these and other questions in a series of experiments.
Do you Really Need to Remove the Silver After 10 Minutes?
For the first experiment, I took a pair of highly tarnished earrings and placed one in the aluminum foil/salt water bath for the standard 10 minutes. I placed the other in a bath for 20 minutes. Except for time, I kept everything else the same: I used the same amount of water for each bath, the same amount of aluminum foil, and the same amount of baking soda.

10 Minutes versus 20 Minutes: Before: Two equally tarnished earrings before being submitted to different water bath times.

10 minutes versus 20 minutes:The two earrings, each in their respective bath, just before water was added.
As you can see from the "After" pictures, there wasn't a much difference between the two earrings, though maybe there was a hint less tarnish after the 20-minute bath. Certainly, the 20-minute earring did not become more tarnished, suggesting that it would be fine to leave silver in the bath for more than 10 minutes.
Do you Really Need Boiling Water?
For the second experiment, I took a second pair of earrings, also highly tarnished. I placed one in a boiling hot baking soda solution, and I placed the other one in a merely warm water baking soda solution (125 degrees out of the tap, in case anyone wonders). As with the first experiment, I kept everything else the same. I used the same amount of water, baking soda, and aluminum foil for both. Having learned that 20 minutes is at least as good as 10, I kept each earring in its respective bath for 20 minutes exactly.

Boiling Water versus Warm Water: Before: again, two equally tarnished earrings. The earring on the left was put into a boiling water bath, whereas the earring on the right was put into warm water.

Boiling Water versus Warm Tap Water: After: The earring placed in boiling water is on the left. The earring placed in warm tap water is on the right. Notice the right earring is still much less tarnished than it was prior to the bath!
As you can see, the earring placed in boiling water is visibly less tarnished than the one placed in warm tap water--but the one placed in warm tap water is still strikingly less tarnished than it was prior to being cleaned. This suggests that the redox reaction still works with warm water, but just not as fast. If you need to avoid boiling water for some reason, go ahead and just use tap water.

In this experiment both earrings came out equally cleaned. I used porcelain on the left and plastic on the right. Notice, though, that both cups are completely lined with aluminum
Do you Really Need to use a Clean Pyrex Dish to Hold Everything?
The rationale for using a clean Pyrex dish to hold your aluminum, water, silver, and baking soda is to avoid introducing other metals into the bath. Other metals might have less affinity for sulfide than silver. When that happens, you can actually increase the tarnish on your silver. If you use a metal pan, the pan might have a greater affinity for sulfide than aluminum. In that case, the pan will become tarnished. When I clean silver I create separate aluminum “boats” for every jewelry piece. Since the water bath never comes in contact with the holding pan, I have found no difference between using Pyrex, plastic, or regular glass. However, even I would never use a metal pan! There's just too great a chance that some unwanted metal ions will get into the saltwater bath.
Does the Aluminum Need to be Shiny-Side up?
No. The shiny v. dull appearance of aluminum is simply a function of the rollers used to press aluminum into foil. The chemical and physical properties of aluminum do not differ according to which side you use. This is true of cooking, by the way. It doesn’t matter whether you cover your turkey with the shiny side up or the dull side up. I didn't bother doing an experiment for this one.
Read More From Bellatory
Does the Aluminum Foil Need to be Crumpled?
It depends. For curved or irregularly shaped silver items, crumpling the foil may increase direct contact between it and the silver piece. This helps speed tarnish removal. For straight smooth pieces, flattened foil may have better direct contact.
Preventing Tarnish and Oxidation
Of course, you would never need to remove silver tarnish if it didn’t accumulate in the first place. In order to prevent tarnish and oxidation, you need to first understand the causes. Tarnish, as mentioned earlier, is the production of silver sulfide on the silver’s surface. This reaction must occur within a thin film of water on the silver’s surface. When the relative humidity reaches 70-80%, as might happen in an outdoors vending event, the tarnish reaction accelerates dramatically. Below 50% relative humidity, tarnish still occurs, but the rate of change is relatively constant.
Chemical impurities in the air, including organic sulfides, nitrogen oxide, and chlorine also accelerate the tarnish reaction. Fortunately, these normally occur at very low concentrations. Salts from human sweat and exposure to chlorine gas from bleach products or swimming pools are a good deal more common and can react with silver to produce silver chloride. Silver chloride is usually white or clear, but can become dark when mixed with soot or dirt.
Although it is neither oxidation nor tarnish, ultraviolet radiation can reduce ionized silver and create fine silver particles that appear black.
Tips for Preventing Tarnish and Oxidation
Now that you know the background causes of tarnish and oxidation, the following tips will make more sense:
- Store your silver pieces in a box away from light and cleaning products.
- Store your silver pieces with silica gel packets to reduce the local humidity.
- Wrap your silver pieces in jeweler’s cloth. This is cloth has been impregnated with fine silver particles. The fine silver reacts with sulfide “before it can reach” your silver.
- To really exclude humidity and prevent tarnish, you can wrap your silver with jeweler’s cloth, throw it in a box with some silica gel packs, and then put the entire contraption in a Pro-Tectant Anti-Tarnish Anti-Rust Plastic Bag. Or a thick plastic baggy (e.g., 2 mil) will do.
- If pollution is a problem in your area, consider also storing your silver with chemical adsorbents, such as zinc oxide or activated carbon.
- Wipe your silver pieces with a soft cloth after each use
- Do not wear silver jewelry when swimming
Lacquering silver will also eliminate surface water, prevent silver’s exposure to micropollutants, and absorb UV radiation. However, all lacquers break down over time and need to be reapplied. Some people may react to the various chemicals in lacquers as well. Rhodium plating is more expensive but potentially longer-lasting. It too eventually needs to be re-done. Unless you have your own plating device, you will need a jeweler to do this for you.
Polishing clothes, jewelry wipes, jewelry dips, and polishing foams physically and chemically remove tarnish molecules (that is, silver sulfide) from your jewelry. If you have a solid silver piece, this slow removal of silver molecules tends not to be a problem. For very thinly plated silver pieces however, there is risk the silver will be completely removed. Since the baking soda/aluminum foil method actually restores silver, I prefer it for plated items.
What if Your Silver is Set With Gemstones?
Most gemstones tolerate the baking soda/hot water bath just fine, including pearls. Gems set with adhesives should not be submitted to this cleaning process. Water soluble adhesives can dissolve, and even water insoluble adhesives can soften and loosen. Gemstones that most authorities recommend against placing in baking soda/hot water include amber and coral. Speaking from personal experience, I second that.
Authorities are more mixed as to whether opals can be submitted to baking soda and hot water, with some saying only Welo opals are at risk and others arguing that no opal should be submitted. Authorities are likewise mixed as to whether turquoise can be submitted to this cleaning process. I do put turquoise into my aluminum/saltwater/hot water baths. It comes out fine, but always needs to be re-polished. My personal recommendation? When in doubt, don't.
Quiz
For each question, choose the best answer. The answer key is below.
- What is your favorite way to clean silver?
- Aluminum foil and salt water bath, baby!
- Rub with jeweler's cloth
- Silver dip
- I prefer to let my silver tarnish to a deep black patina
- Other
Answer Key
- Rub with jeweler's cloth
Acknowledgments
I am indebted to Masamitsu Inaba for his discussion of the chemistry of tarnish production and prevention.
I am also indebted to you for reading my article. If you enjoyed it or learned something new, please leave a comment or rating.
Questions & Answers
Question: I had a very large silver teapot that was badly tarnished. I performed the above steps in a large aluminium stock pot with a ball of foil thrown in (not lining the pot). Now the inside of the pot has turned very dark gray. Should I be worried about cooking in this pot? Is it safe or will I be ingesting bits of silver?
Answer: Unfortunately, your aluminum pot is now corroded and the dark gray may be Aluminum sulfide. (See the redox equation above). I'm afraid I cannot answer if it is safe to use for food any more. I might direct that question to your county extension office.
Question: Can aluminum foil be reused for cleaning or household uses?
Answer: I usually just recycle used aluminum. I probably wouldn't reuse it for any food use (e.g., I wouldn't wrap food in used aluminum)
Question: Does this process hurt the silver or the aluminum?
Answer: This should not tarnish (hurt) the silver or aluminum. However, if your piece has decorative engraving with blackening agent in the grooves or edges to enhance the contrast, the blackening agent (which is just tarnish, basically) will be removed.
Question: My mom left her silver utensils in the baking soda, salt, and vinegar solution so long that the liquids dried. How do I get off the white film that will not budge off the silverware?
Answer: The cleaning solution is *only* supposed to be comprised of baking soda and hot or warm water. I think salt and vinegar could pit your silver. It sounds like you have already tried polishing it. At this point, I might take your utensils to a jeweler for more expert input! Best wishes to you.
Question: What do I do if I use a liquid silver cleaner and the piece ends up with white on it and what looks like sticky residue? Did I remove some of the protective coating?
Answer: I am going to answer a slightly different question, and perhaps it will apply. I find that, when I take some pieces out of the aluminum/baking soda/water bath, they look "raw" and white instead of silvery and mirror-like. When this happens, polishing or buffing the piece with a chamois cloth will bring back the shine. I suppose it is possible that you removed a protective coating. I suggest contacting the manufacturer of the cleaning solution for help.
Question: I did this yesterday, and my jewellery looked fine. Left it on a towel overnight (no liquids!) and now it has brown and black stains all over it. What happened? Can I reverse the damage?
Answer: I am not certain what happened. One thought might be that the jewelry had a substantial amount of a base metal in them. Another thought might be that there is enough humidity and/or air pollution where you live that overnight was all it took to re-tarnish your silver. If you are convinced your jewelry is silver, you could try repeating the foil/aluminum bath, this time towel drying the jewelry thoroughly and then placing it in a thick plastic bag with a silica desiccant. You could also go ahead and try silver polish to see if you can remove the stains.
Question: I have a sterling center place bowl, coffee tea set, and plated antique water pitcher. I’ve had them professionally cleaned and it cost a lot of money. Do you recommend this process if I can find something to submerge it in? A plastic bin? And what do you recommend for a ratio of baking soda and water?
Answer: In theory this process should work. My mother-in-law used it to clean very large silver pieces. She coated the entire surface of whatever she was cleaning with about 1/4" of baking soda. You need enough water to cover the piece, though for large pieces, you could do one half the piece, then turn it over and do the other half.
Question: Some silver cleaning products say items do not need to be in direct contact with aluminum, that the water, with soda (some add salt) creates the transfer, is that true?
Answer: Please don't add salt! The chloride in salt can permanently tarnish and pit silver. I find I get the best results if the aluminum is in close contact with the silver, but you could experiment and see if there is a difference.
Question: Received via email: Hello I would like to begin and start by saying that the baking soda and foil didn't work. My necklace was black and I had just bought it 2 weeks ago. I live in Arizona and yes I do sweat, all that I understand, now I followed the way you had described, but my necklace still did not return to the shine it was when I first bought it. Yes I am gonna go back to the location where I bought my necklace...but I am telling you that IT DIDN'T WORK!!!?
Answer: From this comment, I am not certain if you are saying it didn't return to an untarnished state or if it didn't return to a mirror shine? Depending on how tarnished a piece is, it may need multiple treatments. I have a piece that was deliberately and heavily oxidized. Unfortunately, I don't think I will ever completely remove its tarnish, even after multiple treatments. As I mentioned in the article, I find that the silver emerges from the bath looking a bit "raw" and white. When this happens, it needs to be rebuffed using a buffing cloth to bring back its shine. If local conditions cause a piece to tarnish as quickly as you describe in your note, I might suggest plating your necklace with rhodium to slow down the tarnish. This costs $$$ and has to be redone periodically. I hope this helps.
Comments
Mohammedi Siyam on May 25, 2020:
Can I use this method on my Rhodium played jewellery ?
Does baking soda cause any damage to the rhodium ?
Dell Erwin on February 13, 2020:
Why do some sites say add salt? Some vinegar.
Eleanor Hoague on November 20, 2019:
I just want to thank you for helping me and others understand the process of tarnishing and removing tarnish. Great article!
lauren on May 21, 2018:
thanks so much for posting your results. i just cleaned all my earrings and feel like i have new jewelry!